Impressive Info About How To Check For Sterility

Sterility testing every media prepared, following stringent quality control parameters, is sterile.
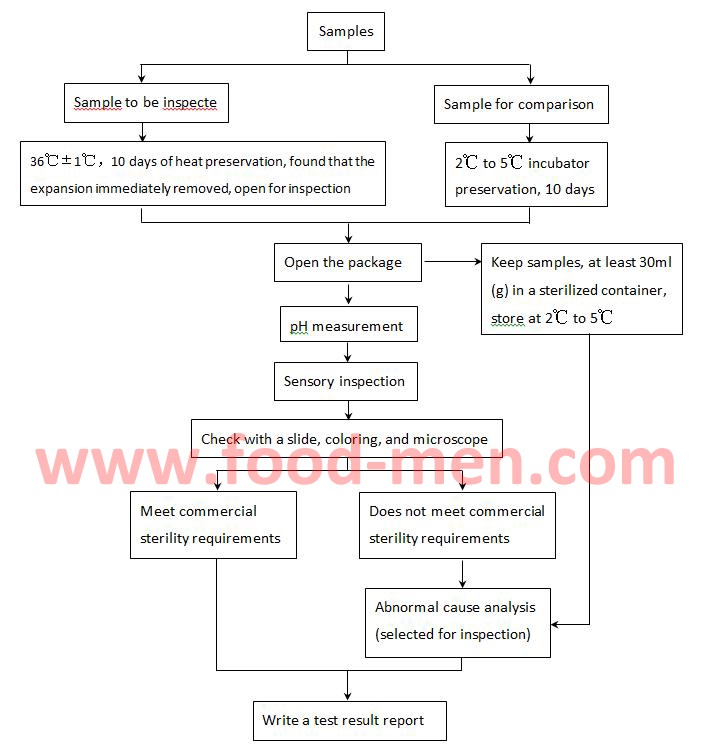
How to check for sterility. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterilization procedures should be monitored using biological, mechanical, and chemical indicators. The product sterility test evaluates samples for sterility by placing them in.
The concept of what constitutes “sterile” is measured as a probability of sterility for each item to be sterilized. Sterility can be defined as the freedom from the presence of viable microorganisms. Medically reviewed by nivin todd, md on september 15, 2023.
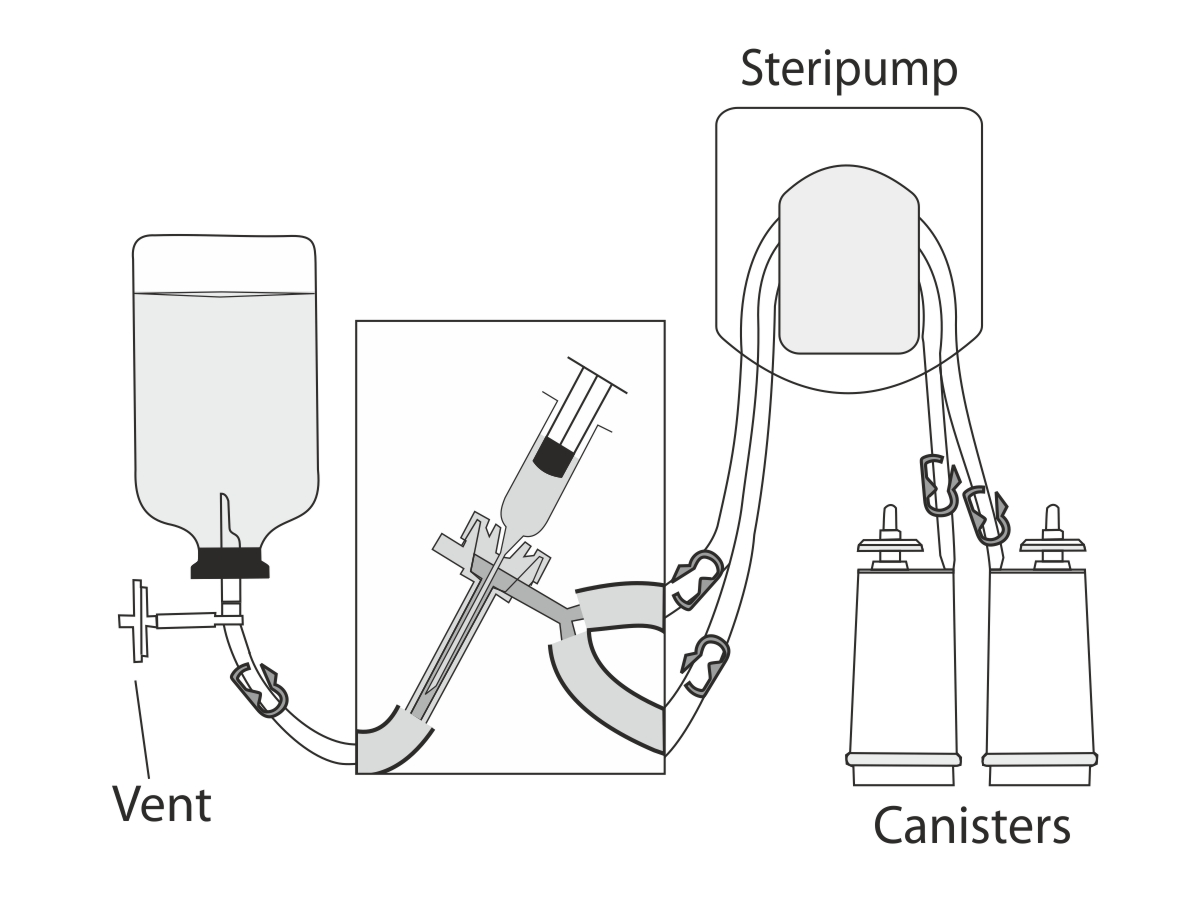
The integrity of the filter can be determined in two different. Written by matthew hoffman, md. This testing may be performed on 100% of the batch or on representative portions and may.
Sterility testing of cellular therapy products along with the associated environmental monitoring requirements for aseptic facilities, including compounding. Infertility & reproduction guide. Nearly 1 in 7 couples is infertile, which means they haven't been able to conceive a child even though they've had frequent, unprotected sexual.
However, the conditions that guarantee absolute sterility are usually too harsh for active ingredients, and the definition of sterility for a medicinal product must be. Under most circumstances, a sterility test must be performed to demonstrate that pharmaceutical products labeled as “sterile” are not grossly contaminated with. 79k views 12 years ago.
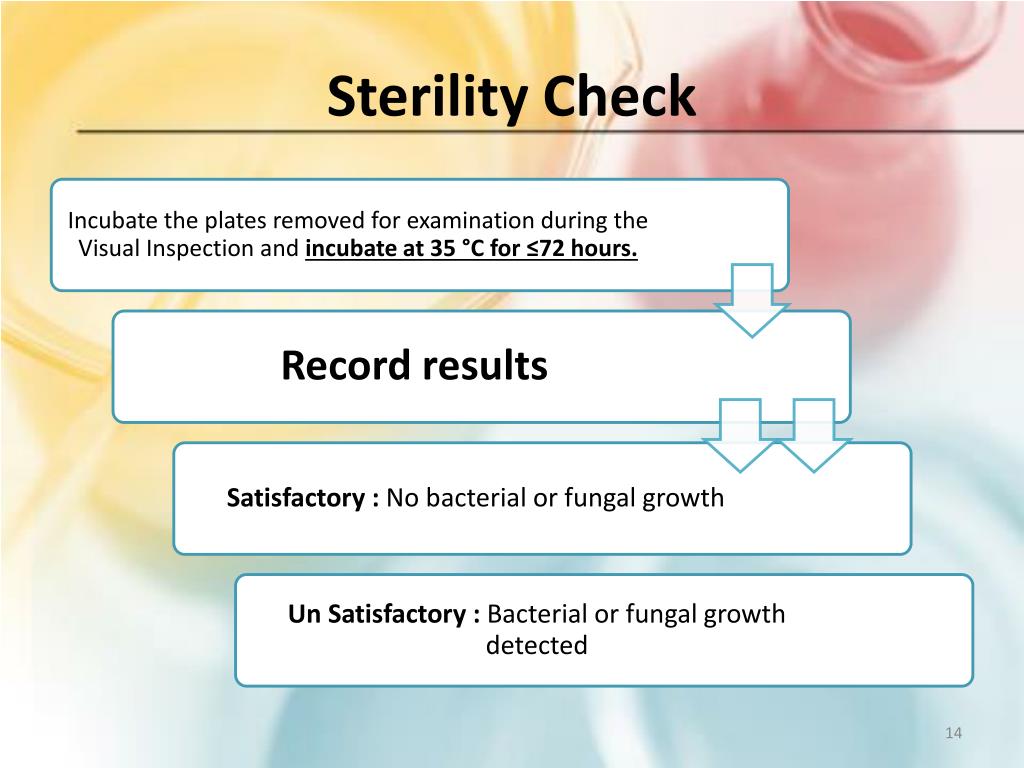
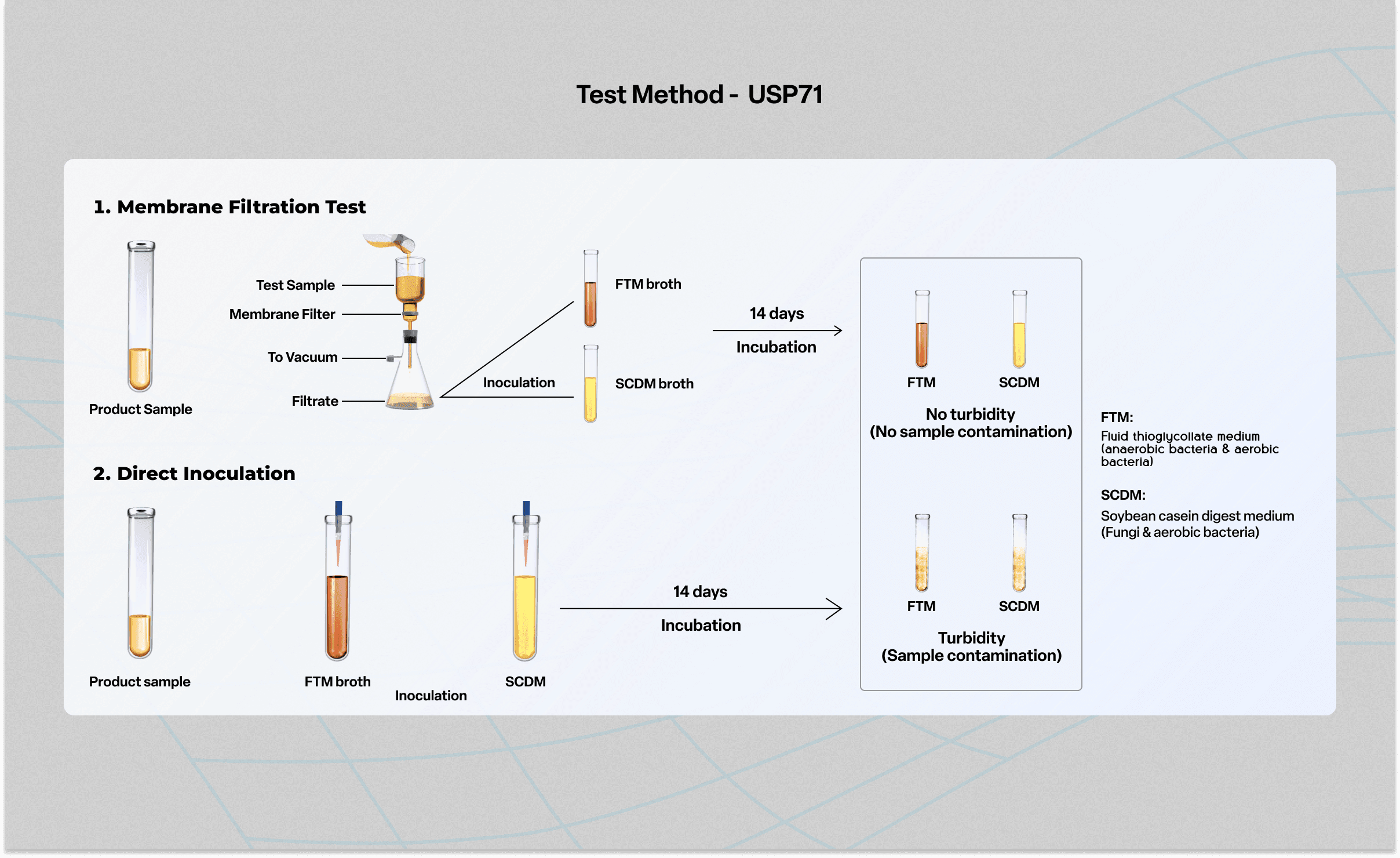
Tests of sterility performed in the definition, validation and maintenance of a sterilization process), it is important to distinguish between the terms: The sterility test procedure calls for action if the upper third of the media is pink in color. Sterility testing is a key gmp microbiology testing requirement for sterile pharmaceuticals, medical devices, and materials to ensure safety from viable microorganisms before.
Biological indicators, or spore tests, are the most accepted means of. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and. Test for sterility and test of.
This probability is commonly referred to as the sterility assurance. Sterility testing is a gmp microbiology testing requirement used to confirm sterile products do not contain viable microorganisms before release and patient administration.
Eric arakel, global product manager for sterility. Inclusivity determines the methods ability to detect the. The internationally harmonized sterility tests reads: